When designing a permanent magnet for a given application, myriad factors must be considered, including material, grade, size, shape, total mass, direction of magnetization, and other aspects. However, the designer must also take into account environmental considerations and consider exposure to strong electromagnetic fields, wild swings in operating and maximum temperature, and, for purposes of this discussion, corrosive environments.
Permanent magnets do not exist within a vacuum (unless, of course, that is their intended use) and are often exposed to corrosive conditions in the field. Some magnetic materials such as Samarium Cobalt are naturally corrosion-resistant and require extra protection only in extreme circumstances. However, Neodymium-Iron-Boron (NdFeB), currently the strongest commercially available magnetic material, is particularly susceptible to rust and requires a protective finish or coating.
NdFeB magnets in most commercial applications are sealed inside another body, e.g. a motor, and are not directly exposed to caustic substances, sprayed water, or even high humidity. Subsequently, industry-standard protective finishes, such as Nickel-Copper-Nickel, provide adequate protection and give these magnets their shiny metallic lustre. Ni-Cu-Ni is not expensive, nor does it negatively impact the magnet’s performance – the very thin layer of protective finish minimizes the air gap.
Ni-Cu-Ni-coated magnets directly exposed to corrosive environments will, however, rust. In addition to unattractive discoloration, as the magnet oxidizes, the magnet loses mass and therefore magnetic flux, weaking the magnetic field and negatively influencing performance. Engineers, designers, and manufacturers must therefore define how to protect against this corrosion. Individual end-user customers may develop their own standards or use public standards like ISO. Most specifications define certain criteria such as:
- maximum surface-area visible rust coverage;
- maximum mass loss;
- maximum magnetic flux, or moment, loss;
These measurements are taken before and after the magnet is exposed to a corrosive environment, for example, a salt-spray chamber with 5% NaCl concentration at a temperature of 35°C for a given number of hours or days. For example, an engineering drawing may dictate “After 17 days of ISO 14993 corrosion testing, part mass shall be >95% of initial mass, magnetic flux shall be >95% of initial magnetic flux when measured at 25°C +/-1◦C, and no more than 10% of the surface area may be covered in rust.” Thus, the designer helps to ensure that the magnet will perform at its intended use for years of service.
Salt Spray and Neodymium Magnets Case Study
A manufacturer of linear power generators asked Bunting for help validating whether a black epoxy coating could withstand the noted 17-day salt spray test when applied to bread-loaf magnets. Normally, this would mean applying a Ni-Cu-Ni finish and then a second black epoxy coat to improve corrosion protection. However, these magnets were laminated – essentially, thin sections of magnetic material glued, or laminated, together to form the bread loaf. The laminating material does not allow for the deposition of the Ni-Cu-Ni finish, so epoxy was the only real option. Applied in thick enough layers, the epoxy is expected to have very good corrosion protection, but sharp corners, edges, and the aforementioned lamination lines all tend to have relative thin coverage, due to how the epoxy coats.
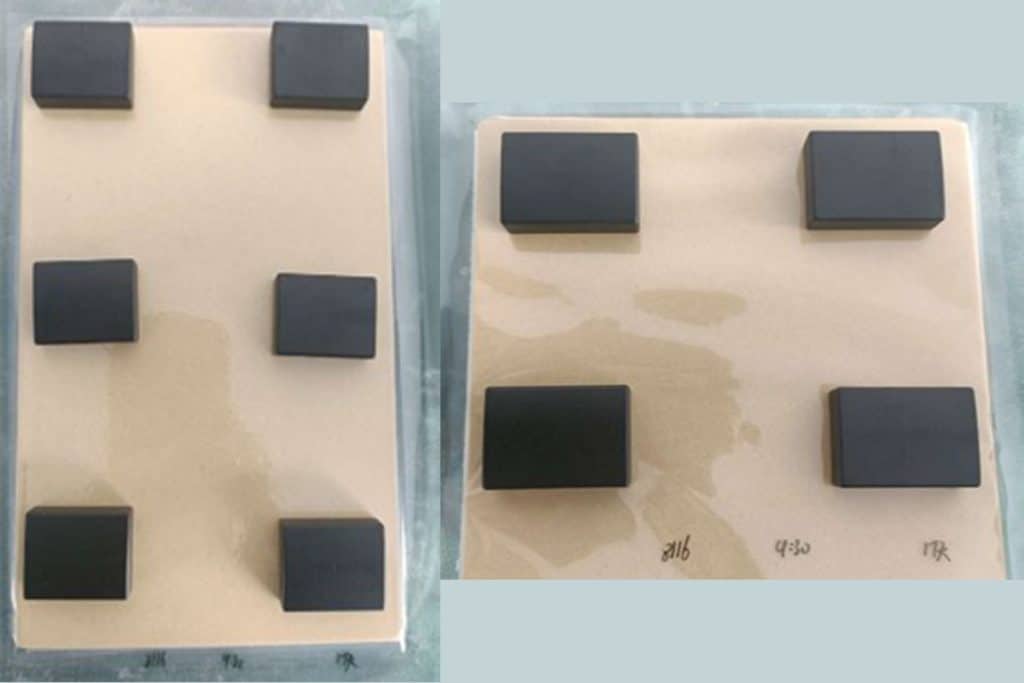
10 N50SH magnets were coated with black epoxy and 10 separate N50SH magnets with Everlube 6155, another common finish. The Everlube is not intended to have strong corrosion protection, so these magnets were a sort of control group, used to show the corrosive nature of the test. The magnets are approximately 29.8mm long x 22.5mm thick x 8.9mm tall, in a breadloaf shape. The measurement of mass and moment took place before the test began, as were photographs to help visualize visible rust progression.
The magnets were then immersed in a salt spray chamber and sprayed continuously with a 5% NaCl solution at 35◦C for 24-hour (1 day) periods. As expected, the Everlube control group exhibited significant rust within the first 24-hour period; none of the 10 samples passed after 48 hours.
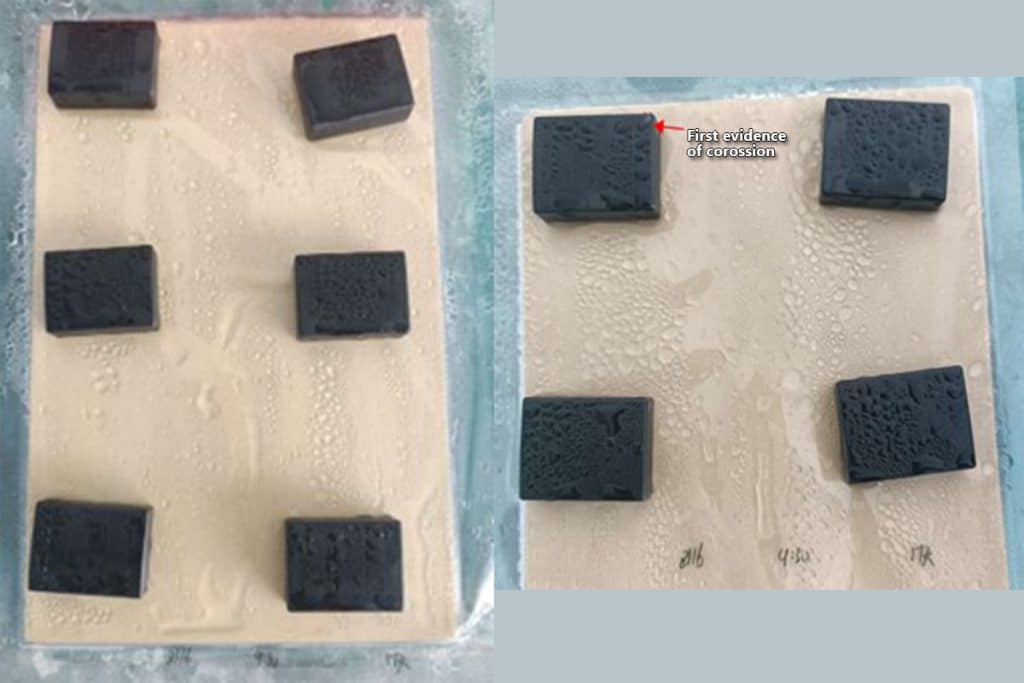
The epoxy-coated magnets, however, exhibited very good corrosion protection. No magnets showed any visible rust after 24 hours. One magnet showed a small spot of rust in a corner after 48 hours. Another magnet showed rust on an edge after 72 hours. After 408 hours/17 days, all ten magnets showed signs of visible rust in single, small spots at the corners or edges, as expected. The rust did not spread from the initial spot, even on the first two magnets that showed it. Overall, the rust coverage was well below the 10% allowable surface area specification.
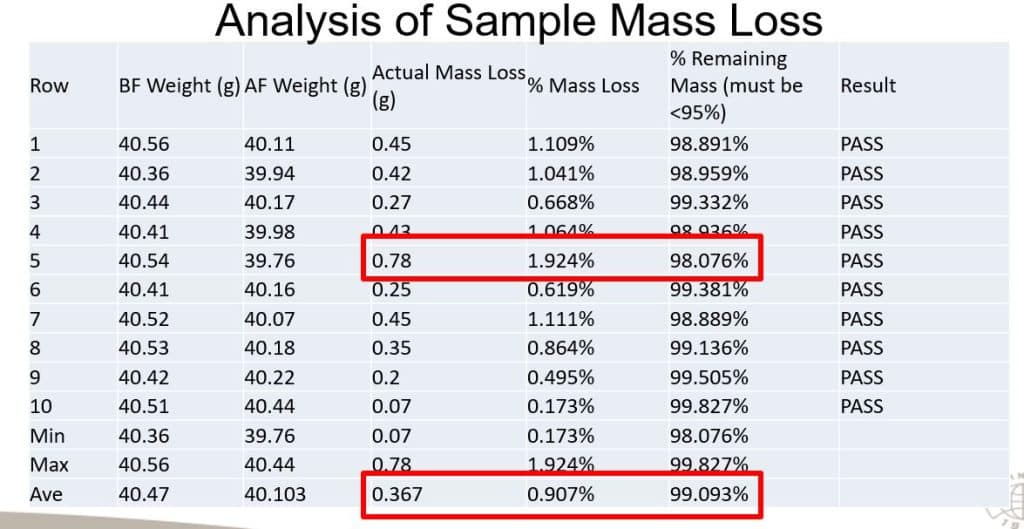
Each magnet did exhibit some degree of mass loss, which is to be expected due to oxidation. The mass loss ranged from 0.07g loss to 0.78g loss, with an average loss of 0.367g across the 10 samples, which works out to about min 0.173%, max 1.924%, and average 0.907% mass loss respectively. Again, this is well below the 5% acceptable loss threshold.
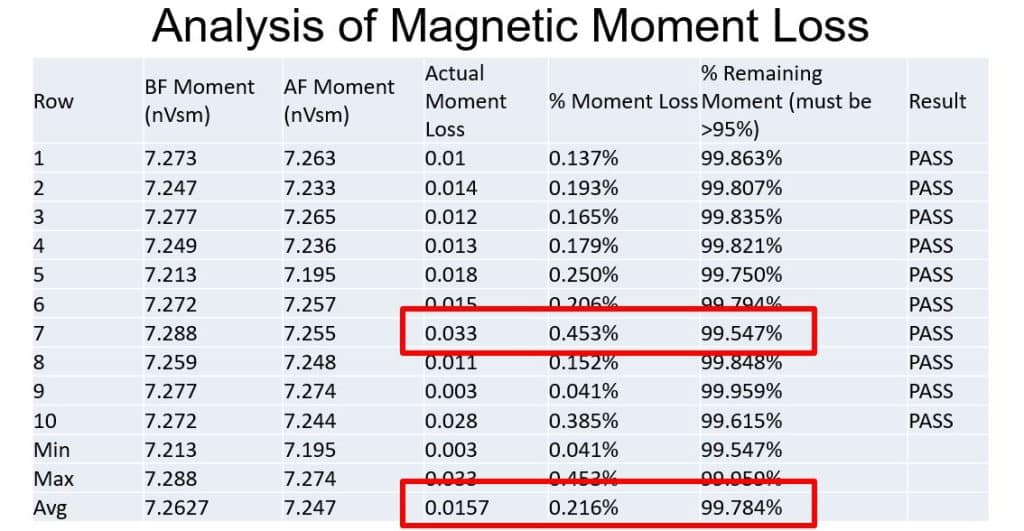
Ultimately the most important part of this study is the magnetic moment loss, which can have a direct impact on performance of the generator. The moment loss is expected to trend with the mass loss, but not be as pronounced, because the lost mass is typically along the grain boundary, which typically does not add to the strength of the magnet. The data bore out this expectation – the worst-case moment loss was 0.033nVsm, or about 0.453%; the best-case moment loss was 0.003nVsm, or about 0.041%; and the average moment loss was 0.0157nVsm, or about 0.216%. Not only did every magnet pass, they did so with exceptional performance against the maximum allowable 5% moment loss.
Conclusion
Black epoxy performed exceptionally well in this particular salt spray test on these particular magnets. Every magnet is different and so a designer should not assume the epoxy will work this well in all applications, but these results are encouraging.
For further information or to discuss a specific application, please contact Bunting’s technical magnetics experts at Bunting.
This article was written by Francis “Mac” Kern, Bunting’s New Business Development Manager in the US Western Region
